Pfizer to continue distributing version of COVID-19 vaccine not fully approved by FDA
Sunday, December 26, 2021 | Tag Cloud Tags: covid-19, EU, News, Worthy News | Learn about our FREE SYNDICATION Service | Sign up for our Worthy Briefs! | Printer Friendly
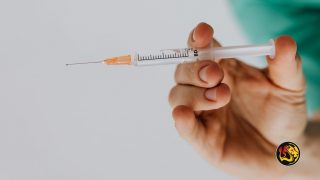
(Worthy News) – Pfizer’s vaccine against COVID-19 has been fully approved by the Food and Drug Administration, yet the pharmaceutical giant is still providing distributors across the country with an earlier version of the vaccine that predates FDA’s full approval.
The Pfizer-BioNTech vaccine allowed under federal Emergency Use Authorization (EUA) in December 2020 and the Comirnaty vaccine approved by the FDA in August are identical, according to Pfizer and several experts.
However, the two vaccines are legally distinct, raising questions over the legality of vaccine mandates. [ Source: Just the News (Read More…) ]
Fair Use Notice:This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a 'fair use' of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond 'fair use', you must obtain permission from the copyright owner.
